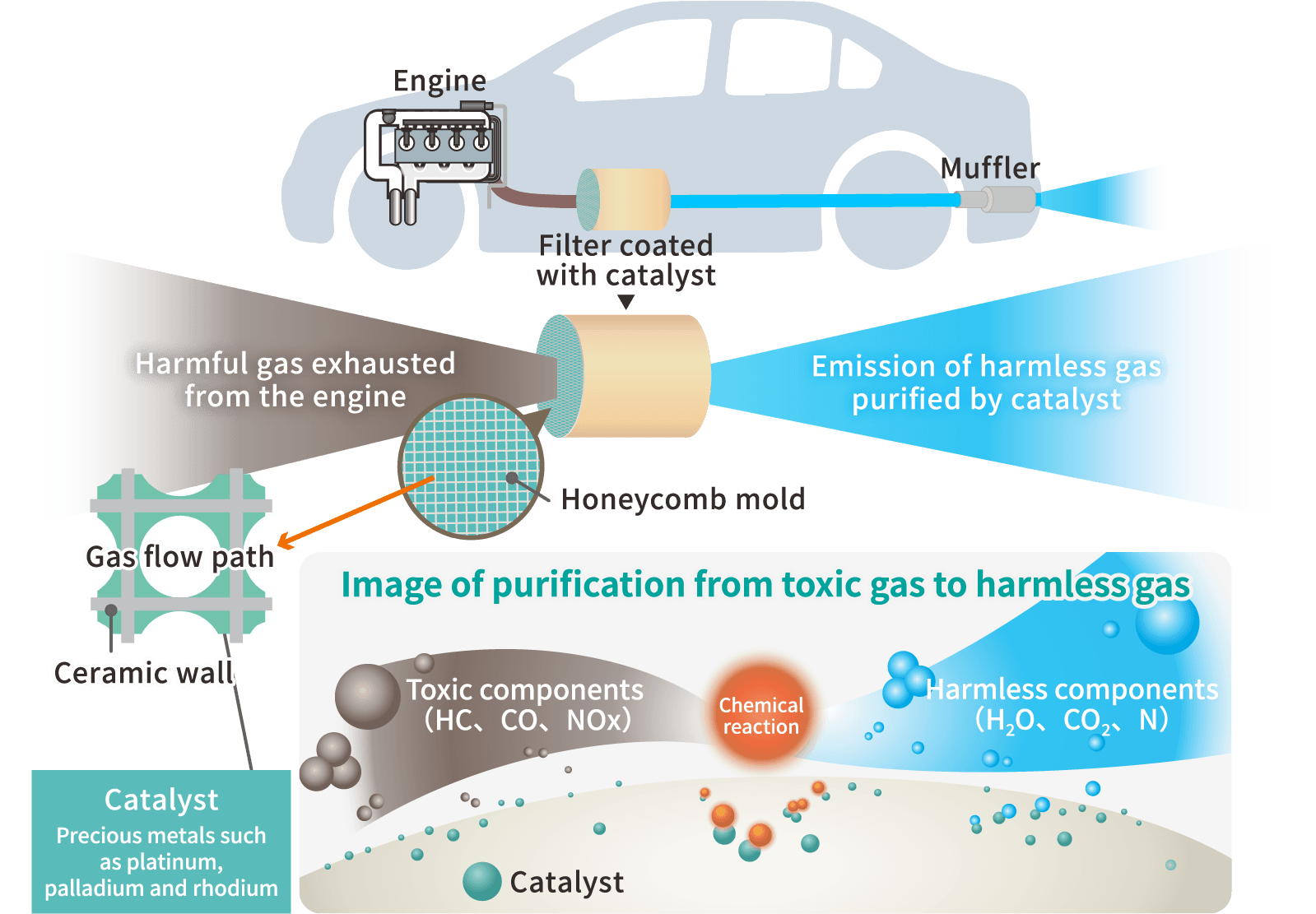
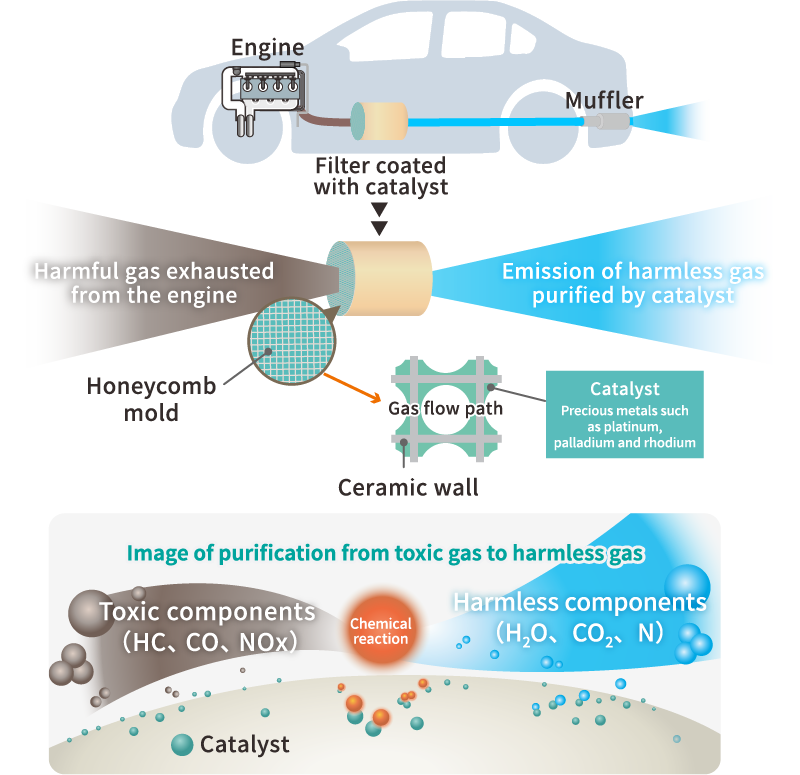
Cataler's catalyst technology
<2016> (5 minutes 35 seconds)
In order to achieve both high performance and cost reduction of automobile exhaust gas purification catalysts, which are Cataler's strengths, we carry out R&D in four fields: precious metal nano-processing, material, coating, and component technologies.


 JAPANESE
JAPANESE

 Select Language
Select Language English
English