Shinka = 共進化・真果 / FCEV
<2025> (2 minutes 31 seconds)
A branding movie to prove "共進化・真果" of Cataler.
共進化・真果 = Collaboration / Inspiration / For the future
The electrocatalyst is a key material that greatly affects the output of the fuel cell stack (FC stack) in fuel cell electric vehicles (FCEV), the "ultimate eco-car", as well as the ratio of hydrogen (H2) consumption to the vehicle's driving range. Cataler's electrocatalysts helped overcome challenges such as increasing the output of the FC stack and improving the H2 consumption rate, and contributed to improving the performance of the world's first mass-produced fuel cell electric vehicle (FCEV), the MIRAI (Toyota Motor Corporation), which was launched in 2014. Cataler's electrocatalysts are also used in the MIRAI, which was fully remodeled in 2020.

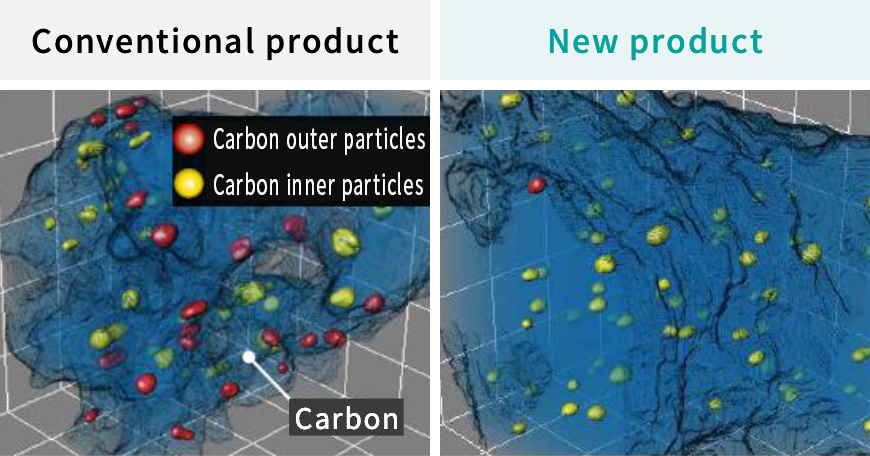
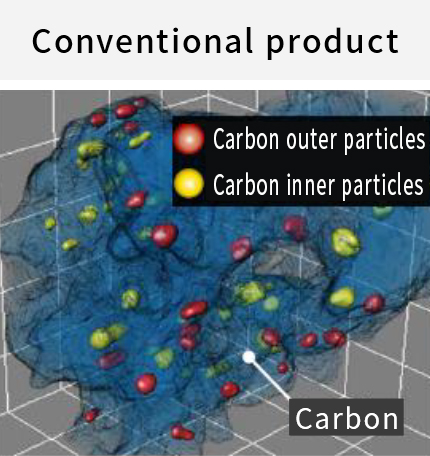
Cataler evolved an electrocatalyst for Toyota Motor Corporation's new MIRAI (2020), and started mass production. The new electro catalyst preferentially places platinum-cobalt alloy particles(PtCo) within the pores of the carbon support(*) to suppress contact between PtCo and ionomer(*). As a result, the catalytic performance was significantly improved compared to conventional electrocatalysts. This performance improvement greatly reduced the amount of platinum used, contributing to the cost reduction of fuel cell electric vehicles (FCEV).


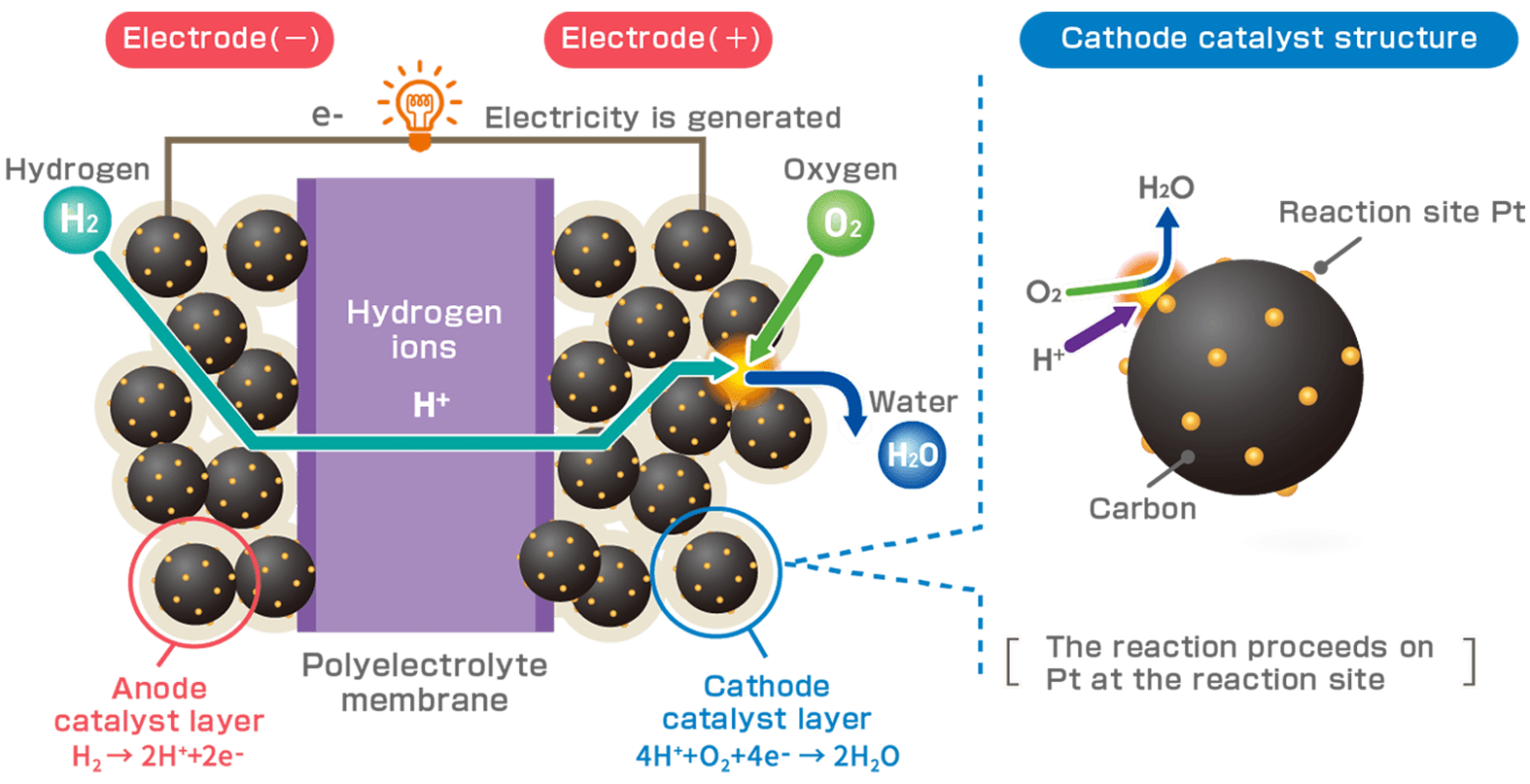
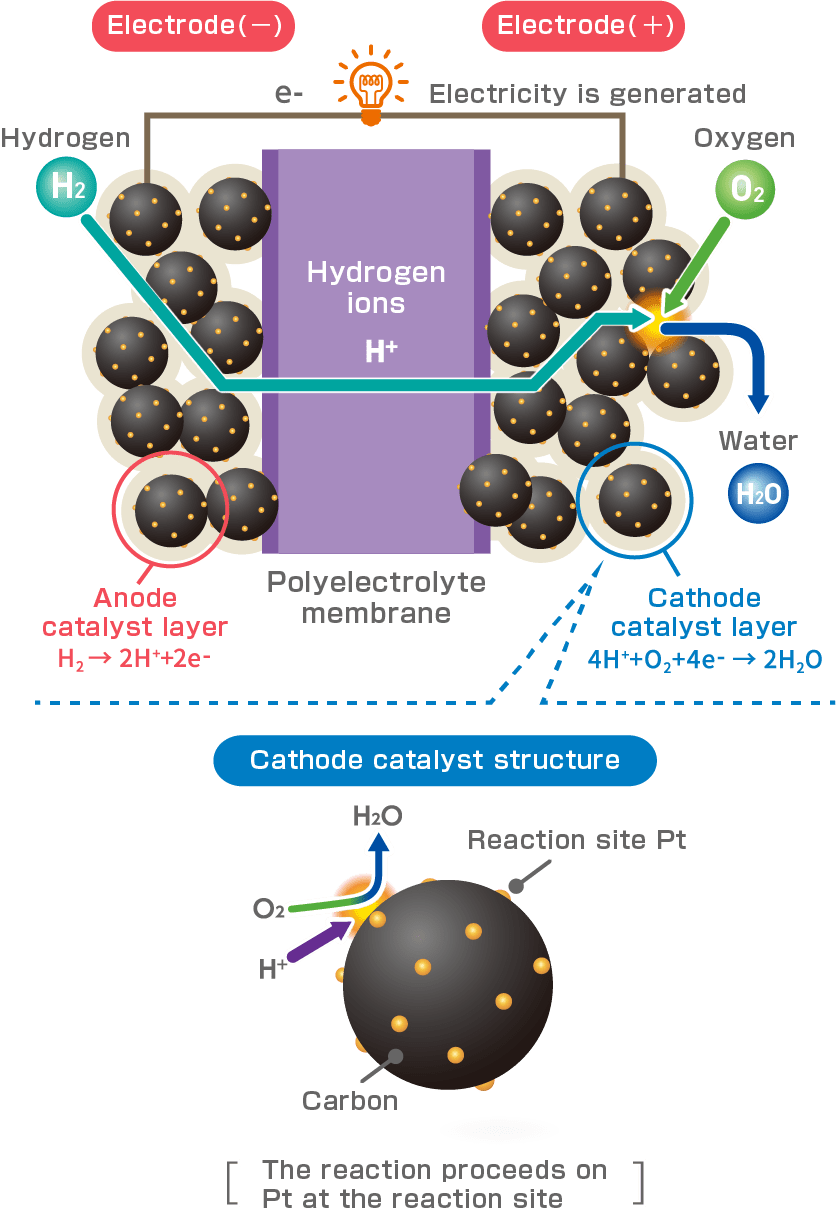
Serves as a support for very high dispersion of platinum-cobalt particles and at the same time acts as an electronic conductor
Polymer electrolyte contained in the catalyst layer. It is responsible for adhesion of the membrane to the catalyst layer and the proton (H+) conductors generated by the chemical reaction.
Decarbonization of lifestyles is essential to the realization of Carbon Neutrality (*). Therefore, various policies to utilize hydrogen (H2) have been established in different countries and regions, and there is a growing need to utilize fuel cell technology as one of the solutions. Fuel cell electric vehicles (FCEV) chemically react H2 with oxygen (O2) in the air to generate electricity and drive motors, so they do not emit carbon dioxide (CO2), and only emit water (H2O). It is a next-generation eco-car. Electrocatalysts play an important role in promoting the reaction between H2 and O2 in the catalyst layer of the fuel cell.
Cataler's electrocatalysts have been continuously used since the first generation "MIRAI" released in 2014.

It means balancing greenhouse gas emissions and absorption. In October 2020, the Japanese government declared its goal of becoming Carbon-Neutral by 2050, reducing greenhouse gas emissions to zero overall.
Click here for details (What is Carbon Neutrality? - Decarbonization Portal | Ministry of the Environment)

A fuel cell electric vehicle (FCEV) is a vehicle equipped with a stack that produces electricity through a chemical reaction between hydrogen (H2) and oxygen (O2), and runs on a motor. By supplying H2 as fuel, fuel cells extract electrical energy through an electrochemical reaction with O2, and use it as electric power. Unlike primary batteries such as dry cell batteries or secondary batteries that are recharged and used repeatedly, fuel cells are "power generators" that can continuously extract electricity. Fuel cell electric vehicles (FCEV) that use hydrogen as fuel are called "ultimate eco-cars" that do not emit the carbon dioxide (CO2) that causes global warming.
Basically, only water (H2O) is emitted while a fuel cell electric vehicle (FCEV) is running. There are no emissions of greenhouse gases (GHG) including carbon dioxide (CO2) or air pollutants including nitrogen oxides (NOx), hydrocarbons (HC), carbon monoxide (CO), and suspended particulate matter (PM). Also, harmful air pollutants such as benzene (C6H6) and acetaldehyde (C2H4O) are not emitted.

It is difficult to stably supply power from renewable energy such as solar power and wind power, but long-term storage is possible by converting the power into hydrogen (H2). Fuel cell electric vehicles (FCEV) can use hydrogen (H2) as fuel, making effective use of renewable energy, while at the same time contributing to reducing environmental impact and becoming carbon-neutral.

Fuel cell electric vehicles (FCEV) use electricity generated by electrochemical reactions to drive motors, so noise can be reduced compared to vehicles that use internal combustion engines (ICE). It is a car that is not only comfortable, but also friendly to the living environment.

It takes about 3 minutes to charge a fuel cell electric vehicle (FCEV) with hydrogen (H2). Unlike electric vehicles (BEV), which require long charging times, fuel cell electric vehicles (FCEV) can be recharged in a short time, similar to gasoline internal combustion engine (ICE) vehicles. In addition, the mileage per charge is 630-850km, which is longer than that of BEVs, and will be on par with gasoline internal combustion engine (ICE) vehicles in the future.

Vehicles equipped with a function that can supply power to an external source can be used as an emergency power supply in the event of a disaster.

Unlike cars that use internal combustion engines (ICE), fuel cell electric vehicles (FCEV) run on electricity generated by a chemical reaction between hydrogen (H2) and oxygen (O2) to power the motor. H2 stored in the hydrogen tank combines with O2 in the fuel cell (FC stack) to generate electricity, which turns the motor to run the car.
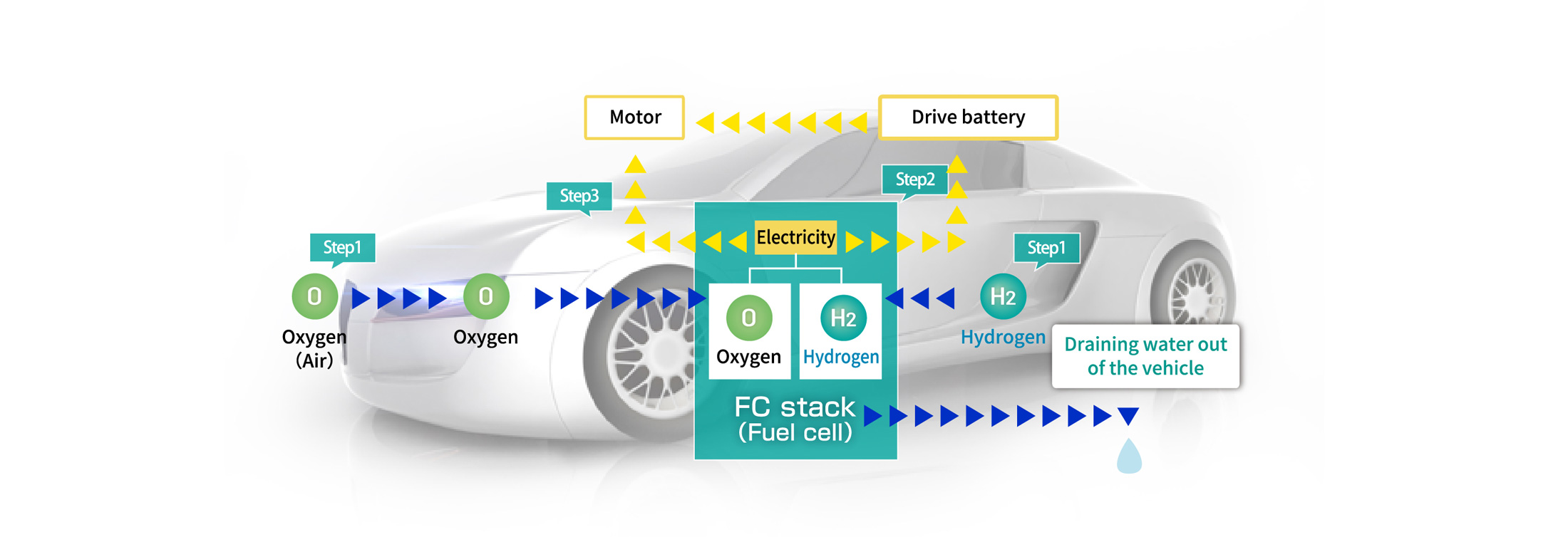


Fuel cell electric vehicles is loaded with hydrogen as fuel, and is supplied with oxygen from the atmosphere.
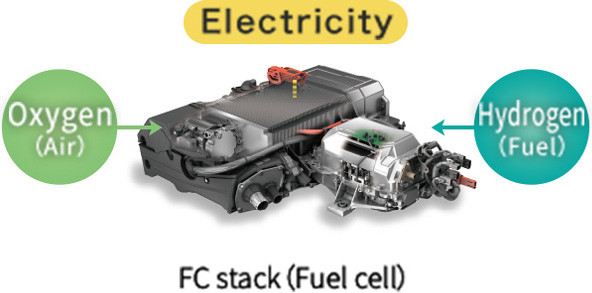
"Electrical energy" is extracted by chemically reacting hydrogen and oxygen
Photo of FC stack courtesy of Toyota Motor Corporation
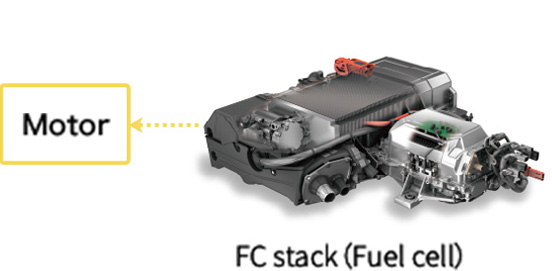
Power is sent from the FC stack to the motor to start the car
Photo of FC stack courtesy of Toyota Motor Corporation
Swipe sideways to see the table.
The part that generates electricity in a fuel cell electric vehicle (FCEV) is called an FC stack. This FC stack consists of an assembly of cells in which MEA (*) are sandwiched between separators (*).
Each separator are formed with channels for hydrogen (H2) as fuel and oxygen (O2) from the outside air. H2 is supplied to the fuel electrode (negative electrode / anode catalyst layer) and O2 to the air electrode (positive electrode / cathode catalyst layer) of the MEA, which generates electricity through an electrochemical reaction. Each electrode contains an electrocatalyst, which plays an important role in promoting the reaction between H2 and O2.
Photo of FC stack courtesy of Toyota Motor Corporation
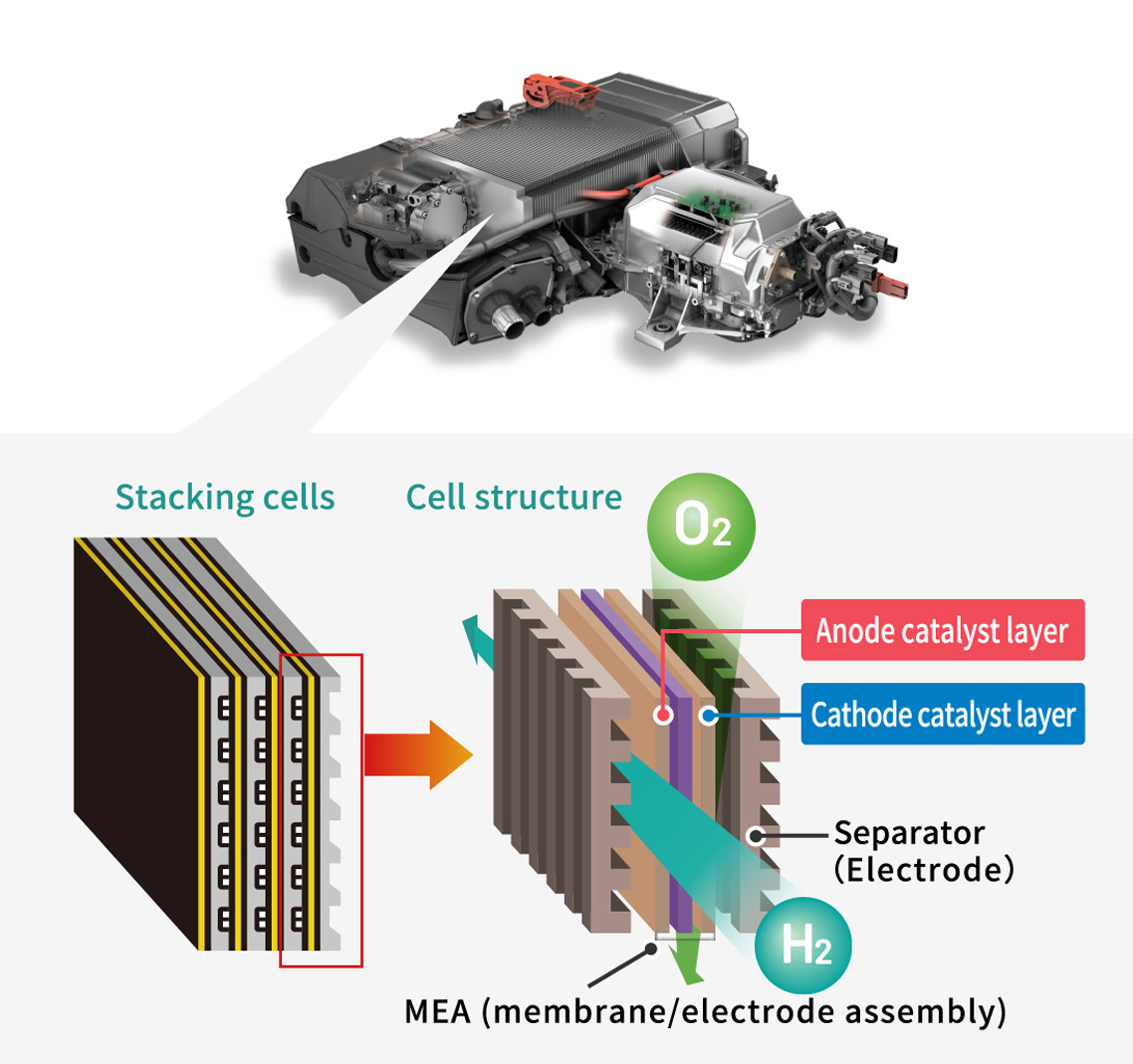
A plate-like component with channels through which hydrogen (H2) or oxygen (O2) flows.
It consists of an electrode (catalyst layer) comprising an electrolyte and a catalyst, an electrolyte membrane, and a gas diffusion layer.